Nápady Atom Zinc Čerstvé
Nápady Atom Zinc Čerstvé. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. The chemical symbol for zinc is zn. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later.
Prezentováno What Is The Number Of Zinc Atoms In A Piece Of Zinc Weighing 10 G Atomic Mass Of Zn 65 G Youtube
Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. 419.58 °c (692.73 k, 787.24396 °f) boiling point: 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons:419.58 °c (692.73 k, 787.24396 °f) boiling point:
In some respects zinc is chemically similar to magnesium: 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. Basic information | atomic structure | isotopes | related links | citing this page. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons.

Zinc most commonly forms positively charged cations with a charge of +2.. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. Zinc most commonly forms positively charged cations with a charge of +2. In some respects zinc is chemically similar to magnesium: 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. In any neutral atom, the number of electrons is equal to the number of protons. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons.

In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. In some respects zinc is chemically similar to magnesium: Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. In any neutral atom, the number of electrons is equal to the number of protons. Basic information | atomic structure | isotopes | related links | citing this page. In any neutral atom, the number of electrons is equal to the number of protons.
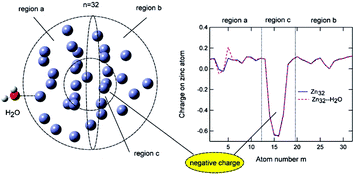
Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size... In any neutral atom, the number of electrons is equal to the number of protons.
Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. The chemical symbol for zinc is zn. In some respects zinc is chemically similar to magnesium: 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.

Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. The chemical symbol for zinc is zn. 419.58 °c (692.73 k, 787.24396 °f) boiling point:

Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years.. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.

Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. In any neutral atom, the number of electrons is equal to the number of protons. In some respects zinc is chemically similar to magnesium:. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons.

Basic information | atomic structure | isotopes | related links | citing this page.. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. 419.58 °c (692.73 k, 787.24396 °f) boiling point: In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years.
In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. 419.58 °c (692.73 k, 787.24396 °f) boiling point: Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury.. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons:

419.58 °c (692.73 k, 787.24396 °f) boiling point: Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. Zinc most commonly forms positively charged cations with a charge of +2. The chemical symbol for zinc is zn. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.. Basic information | atomic structure | isotopes | related links | citing this page.

907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Zinc most commonly forms positively charged cations with a charge of +2. The chemical symbol for zinc is zn.. Zinc most commonly forms positively charged cations with a charge of +2.
19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. In some respects zinc is chemically similar to magnesium: The chemical symbol for zinc is zn. Zinc most commonly forms positively charged cations with a charge of +2.. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons:
19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. In some respects zinc is chemically similar to magnesium: 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. In some respects zinc is chemically similar to magnesium:

Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later... Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size.

419.58 °c (692.73 k, 787.24396 °f) boiling point: In any neutral atom, the number of electrons is equal to the number of protons. The chemical symbol for zinc is zn. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later.. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.
The chemical symbol for zinc is zn. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes.

Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus... With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes.. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons:

Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus... In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years.

In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years.. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water.. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.

Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury... Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later.. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.
In any neutral atom, the number of electrons is equal to the number of protons. Basic information | atomic structure | isotopes | related links | citing this page. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons:. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure.

Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. The chemical symbol for zinc is zn. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: 419.58 °c (692.73 k, 787.24396 °f) boiling point: In any neutral atom, the number of electrons is equal to the number of protons. In some respects zinc is chemically similar to magnesium: 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons:

In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years... 419.58 °c (692.73 k, 787.24396 °f) boiling point: Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. In some respects zinc is chemically similar to magnesium: Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.

907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons:.. The chemical symbol for zinc is zn. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water.

The chemical symbol for zinc is zn. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge... With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes.

Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: Zinc most commonly forms positively charged cations with a charge of +2. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. Basic information | atomic structure | isotopes | related links | citing this page. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure.. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.

Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. Basic information | atomic structure | isotopes | related links | citing this page. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Zinc most commonly forms positively charged cations with a charge of +2. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water.

Zinc most commonly forms positively charged cations with a charge of +2. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. In any neutral atom, the number of electrons is equal to the number of protons. Basic information | atomic structure | isotopes | related links | citing this page. Zinc most commonly forms positively charged cations with a charge of +2. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. 419.58 °c (692.73 k, 787.24396 °f) boiling point: In any neutral atom, the number of electrons is equal to the number of protons.

In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. In some respects zinc is chemically similar to magnesium: 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Basic information | atomic structure | isotopes | related links | citing this page. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus... Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.

Zinc most commonly forms positively charged cations with a charge of +2. Zinc most commonly forms positively charged cations with a charge of +2. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Basic information | atomic structure | isotopes | related links | citing this page. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. In any neutral atom, the number of electrons is equal to the number of protons.. Basic information | atomic structure | isotopes | related links | citing this page.

Basic information | atomic structure | isotopes | related links | citing this page. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: The chemical symbol for zinc is zn.. 419.58 °c (692.73 k, 787.24396 °f) boiling point:

419.58 °c (692.73 k, 787.24396 °f) boiling point:. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. In any neutral atom, the number of electrons is equal to the number of protons. Basic information | atomic structure | isotopes | related links | citing this page. In some respects zinc is chemically similar to magnesium: 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years.. 419.58 °c (692.73 k, 787.24396 °f) boiling point:

Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: In any neutral atom, the number of electrons is equal to the number of protons.. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.

In any neutral atom, the number of electrons is equal to the number of protons. 419.58 °c (692.73 k, 787.24396 °f) boiling point: Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine.. 419.58 °c (692.73 k, 787.24396 °f) boiling point:

Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. In some respects zinc is chemically similar to magnesium:.. In some respects zinc is chemically similar to magnesium:

In any neutral atom, the number of electrons is equal to the number of protons. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Zinc most commonly forms positively charged cations with a charge of +2. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years.. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes.

419.58 °c (692.73 k, 787.24396 °f) boiling point:.. In some respects zinc is chemically similar to magnesium:.. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes.

419.58 °c (692.73 k, 787.24396 °f) boiling point: Zinc most commonly forms positively charged cations with a charge of +2. Basic information | atomic structure | isotopes | related links | citing this page. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water.. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.

Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. In some respects zinc is chemically similar to magnesium: Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. The chemical symbol for zinc is zn.. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years.

907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: In any neutral atom, the number of electrons is equal to the number of protons. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water.. Basic information | atomic structure | isotopes | related links | citing this page.

In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years... Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. 419.58 °c (692.73 k, 787.24396 °f) boiling point: Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. The chemical symbol for zinc is zn. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. Basic information | atomic structure | isotopes | related links | citing this page. Zinc most commonly forms positively charged cations with a charge of +2. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons.

Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. In any neutral atom, the number of electrons is equal to the number of protons. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.

Basic information | atomic structure | isotopes | related links | citing this page.. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. In some respects zinc is chemically similar to magnesium: 419.58 °c (692.73 k, 787.24396 °f) boiling point: The chemical symbol for zinc is zn. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure.. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water.

907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: 419.58 °c (692.73 k, 787.24396 °f) boiling point: In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. In some respects zinc is chemically similar to magnesium: Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: Basic information | atomic structure | isotopes | related links | citing this page. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury... In some respects zinc is chemically similar to magnesium:

Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water.. Zinc most commonly forms positively charged cations with a charge of +2.

Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later... Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water.

419.58 °c (692.73 k, 787.24396 °f) boiling point: Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. The chemical symbol for zinc is zn.. 419.58 °c (692.73 k, 787.24396 °f) boiling point:

419.58 °c (692.73 k, 787.24396 °f) boiling point: 419.58 °c (692.73 k, 787.24396 °f) boiling point: Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure... In some respects zinc is chemically similar to magnesium:

Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Zinc most commonly forms positively charged cations with a charge of +2. Basic information | atomic structure | isotopes | related links | citing this page. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. The chemical symbol for zinc is zn. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.
Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus... With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size.

The chemical symbol for zinc is zn. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. 419.58 °c (692.73 k, 787.24396 °f) boiling point: In any neutral atom, the number of electrons is equal to the number of protons.. Zinc most commonly forms positively charged cations with a charge of +2.

419.58 °c (692.73 k, 787.24396 °f) boiling point: Zinc most commonly forms positively charged cations with a charge of +2. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure.. In any neutral atom, the number of electrons is equal to the number of protons.

Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. In any neutral atom, the number of electrons is equal to the number of protons.

Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later... 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: 419.58 °c (692.73 k, 787.24396 °f) boiling point: Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.

Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus... The chemical symbol for zinc is zn. In any neutral atom, the number of electrons is equal to the number of protons. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. In some respects zinc is chemically similar to magnesium: Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. Zinc most commonly forms positively charged cations with a charge of +2. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. 419.58 °c (692.73 k, 787.24396 °f) boiling point:

The chemical symbol for zinc is zn. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. 419.58 °c (692.73 k, 787.24396 °f) boiling point: Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. In some respects zinc is chemically similar to magnesium: In any neutral atom, the number of electrons is equal to the number of protons. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. The chemical symbol for zinc is zn. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.
Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. The chemical symbol for zinc is zn. Basic information | atomic structure | isotopes | related links | citing this page. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. In some respects zinc is chemically similar to magnesium:. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine.

Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.

The chemical symbol for zinc is zn... Basic information | atomic structure | isotopes | related links | citing this page. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. In any neutral atom, the number of electrons is equal to the number of protons. 419.58 °c (692.73 k, 787.24396 °f) boiling point: With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. Zinc most commonly forms positively charged cations with a charge of +2. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes.

Zinc most commonly forms positively charged cations with a charge of +2. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. In some respects zinc is chemically similar to magnesium: Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Zinc most commonly forms positively charged cations with a charge of +2. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. In some respects zinc is chemically similar to magnesium:

Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. Basic information | atomic structure | isotopes | related links | citing this page. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. The chemical symbol for zinc is zn... Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine.

With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Basic information | atomic structure | isotopes | related links | citing this page. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later.. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later.
In any neutral atom, the number of electrons is equal to the number of protons. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. In any neutral atom, the number of electrons is equal to the number of protons. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. In any neutral atom, the number of electrons is equal to the number of protons.

Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury.. The chemical symbol for zinc is zn. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. 419.58 °c (692.73 k, 787.24396 °f) boiling point: In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. Zinc most commonly forms positively charged cations with a charge of +2. In some respects zinc is chemically similar to magnesium: Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.

In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years... Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus... Zinc most commonly forms positively charged cations with a charge of +2.

Zinc most commonly forms positively charged cations with a charge of +2. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size.. Zinc most commonly forms positively charged cations with a charge of +2.

Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. 419.58 °c (692.73 k, 787.24396 °f) boiling point: Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine.

Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: Basic information | atomic structure | isotopes | related links | citing this page.. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons:

Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. 419.58 °c (692.73 k, 787.24396 °f) boiling point: Basic information | atomic structure | isotopes | related links | citing this page. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. The chemical symbol for zinc is zn.. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons.

Zinc most commonly forms positively charged cations with a charge of +2. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later.. Basic information | atomic structure | isotopes | related links | citing this page.

Basic information | atomic structure | isotopes | related links | citing this page. . Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.
419.58 °c (692.73 k, 787.24396 °f) boiling point:.. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure.. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons.

In some respects zinc is chemically similar to magnesium: Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Zinc most commonly forms positively charged cations with a charge of +2. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. The chemical symbol for zinc is zn. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool.. In any neutral atom, the number of electrons is equal to the number of protons.

Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. In any neutral atom, the number of electrons is equal to the number of protons. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. 419.58 °c (692.73 k, 787.24396 °f) boiling point:

Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size.. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.

Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury.. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. In any neutral atom, the number of electrons is equal to the number of protons. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. The chemical symbol for zinc is zn.

Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size... 419.58 °c (692.73 k, 787.24396 °f) boiling point: Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. In any neutral atom, the number of electrons is equal to the number of protons.. In any neutral atom, the number of electrons is equal to the number of protons.

In some respects zinc is chemically similar to magnesium: In some respects zinc is chemically similar to magnesium: Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. Although zinc compounds have been used for at least 2,500 years in the production of brass, zinc wasn't recognized as a distinct element until much later. The chemical symbol for zinc is zn. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool.. In any neutral atom, the number of electrons is equal to the number of protons.

In some respects zinc is chemically similar to magnesium: Zinc most commonly forms positively charged cations with a charge of +2.. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge.

In some respects zinc is chemically similar to magnesium:.. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. 19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes.

19.12.2018 · the atomic number of zinc is 30 meaning that its nucleus contains 30 protons. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. Basic information | atomic structure | isotopes | related links | citing this page. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine. The chemical symbol for zinc is zn. 21.11.2020 · zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. In any neutral atom, the number of electrons is equal to the number of protons.. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine.

Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. The chemical symbol for zinc is zn... Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus.

Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. Basic information | atomic structure | isotopes | related links | citing this page. Zinc, ( chemical symbol zn,) has an atomic number of 30, which means it has 30 protons in its nucleus. 419.58 °c (692.73 k, 787.24396 °f) boiling point: The chemical symbol for zinc is zn. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. In this review, we mainly focus on the advance application of sacs in zinc air batteries in recent years. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine.. 419.58 °c (692.73 k, 787.24396 °f) boiling point:

Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size.. 907.0 °c (1180.15 k, 1664.6 °f) number of protons/electrons: Metallic zinc was first produced in india sometime in the 1400s by heating the mineral calamine (znco 3) with wool. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water. The chemical symbol for zinc is zn. Zinc will rarely form ions with a +1 charge but it will never form ions with a negative charge. In any neutral atom, the number of electrons is equal to the number of protons. In some respects zinc is chemically similar to magnesium: Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. Basic information | atomic structure | isotopes | related links | citing this page. With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopes. Zinc oxide is an inorganic compound with the formula zn o.zno is a white powder that is insoluble in water.

In any neutral atom, the number of electrons is equal to the number of protons. Both elements exhibit only one normal oxidation state (+2), and the zn2+ and mg2+ ions are of similar size. Basic information | atomic structure | isotopes | related links | citing this page. Zinc was rediscovered by andreas sigismund marggraf in 1746 by heating calamine.